Fédération
Galileo Galilei de Grenoble
Mécanique et procédés
Fédération
Galileo Galilei de Grenoble
Mécanique et procédés
A la une
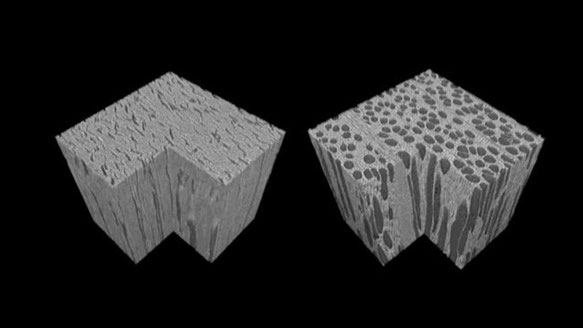
La fed3G regroupe les laboratoires grenoblois
de la mécanique et du génie des procédés
LABORATOIRES
en mécanique des fluides et des solides, génie des procédés et physique de la matière molle
CHERCHEURS
enseignants chercheurs, ingénieurs, techniciens, et personnels administratifs
POST-DOCS
et thèses en moyenne sur l'année
STAGES
de master recherche
%
de l'activité en collaboration avec l'industrie
Vous soutenir

Rencontres scientifiques

Equipements communs
Equipements communs

Plateforme d'innovation
Plateforme d'innovation

Opérations inter-labos
Opérations inter-labos


Evènements, conférences
Evènements, conférences